Stern Electronics Pty. Ltd.
est. 1973 A.C.N 010695360
est. 1973 A.C.N 010695360

These CD’s have a number of features that have meant:
CD use is restricted to hospital inpatients (when they should be used during the period from
immobility to the return to full ambulation)
They are not used when the patient is out of bed (the Kendall SCD Express and Active Care
DVT were intended to address this issue)
Compliance in hospitals by patients and staff is low; “The paramount problem with CD is lack
of compliance”
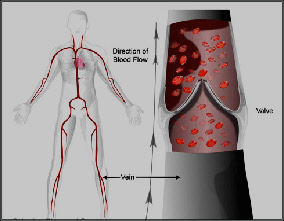
Pneumatic compression devices are mainly used in hospitals and consist of an inflatable cuff or sleeve
wrapped around the arm, leg or foot and an electrical pneumatic pump that inflates the cuff with air
compressing the deep veins and displacing blood proximally, assuming the presence of competent venous
valves. The veins refill from distal flows when the cuffs deflate and thereby the PCD stimulate and maintain
pulsatile blood flow in the deep veins.
There are a number of compression devices (CD’s) developed for hospital use.
These CD’s vary in a number of parameters including:
These CD’s vary in a number of parameters including:
sequential or uniform compression
cuff/sleeve position on the leg or arm
duration of activation (inflation and deflation cycle time)
number of chambers/cells in the cuff/sleeve (single or multiple)
rate of pressure rise (inflation rate)
maximum pressure achieved
CD Features
The Key Features of the I-Cuff
The inventors looked at the currently available sequential
compression devices and saw the huge market potential
both within the existing hospital market and in the un-tapped
non-hospital market of a CD that is:
Light and comfortable to wear
Aesthetically pleasing
Ease of application
Portable
Applies pressure sequentially rather than uniformly
Remotely controlled by a small hand transmitter
compression devices and saw the huge market potential
both within the existing hospital market and in the un-
Aesthetically pleasing
Ease of application
Portable
Applies pressure sequentially rather than uniformly
Remotely controlled by a small hand transmitter